CDC Health Advisory: Elevated Influenza Activity
This is an official
CDC HEALTH ADVISORY
Distributed via the CDC Health Alert Network
January 10, 2020
CDC HAN-00425
Elevated Influenza Activity: Influenza B/Victoria and A(H1N1)pdm09 Viruses are the Predominant Viruses
The Centers for Disease Control and Prevention reminds clinicians that influenza B viruses can cause severe illness in people of all ages, including children. CDC continues to recommend influenza vaccination and prompt antiviral treatment of high- risk outpatients and hospitalized patients with suspected influenza.
Summary
This health advisory notifies clinicians that influenza activity remains high in the United States. Ongoing elevated activity is due to influenza B/Victoria viruses, increasing circulation of influenza A(H1N1)pdm09 viruses, and low levels of influenza B/Yamagata and influenza A(H3N2) viruses. CDC’s influenza forecasts suggest that national influenza activity will remain elevated for several more weeks. Because influenza activity is elevated and both influenza A and B virus infections can cause severe disease and death, this health advisory also serves as a reminder that early treatment with antiviral medications improves outcomes in patients with influenza. Early treatment with antiviral medications is recommended for hospitalized patients and high-risk outpatients, including children younger than two years. Clinicians should continue efforts to vaccinate patients for as long as influenza viruses are circulating, and promptly start antiviral treatment of severely ill and high-risk patients with suspected influenza without waiting for laboratory confirmation.
Background
In the United States, influenza activity remains elevated and widespread, and the season is likely to last several more weeks (see CDC FluView report for details: https://www.cdc.gov/flu/weekly/index.htm).
Since early this season, influenza B viruses, specifically B/Victoria viruses, have been reported more frequently than other influenza viruses, followed by A(H1N1)pdm09. Different viruses have predominated in different parts of the country and among different age groups. Influenza B viruses can cause severe illness in people of all ages, including children.1 In past seasons, the percentage of influenza B viruses detected in children who died with influenza has generally been higher than the percentage of B viruses detected in the general pediatric population,2,3 and pediatric mortality from influenza B–associated hospitalizations has been reported to be higher than with influenza A–associated hospitalizations.4 So far this season, influenza B virus infections account for about half of hospitalizations reported through CDC’s laboratory-confirmed influenza hospitalization surveillance network and the majority of reported influenza- associated pediatric deaths (https://www.cdc.gov/flu/weekly/index.htm). Influenza A(H1N1)pdm09 viruses are increasing and becoming predominant in some regions. These viruses can also cause severe illness, particularly in adults not originally exposed to currently circulating A(H1N1)pdm09 viruses.5 Influenza A(H1N1)pdm09 viruses currently comprise the majority of the other half of hospitalizations reported through CDC’s laboratory-confirmed influenza hospitalization surveillance network.
CDC continues to recommend everyone six months of age and older get vaccinated for influenza. CDC also recommends antiviral medications for the treatment of influenza, because antiviral treatment has shown clinical and public health benefit in reducing illness and lessening severe outcomes of influenza based on evidence from randomized controlled trials, meta-analyses of randomized controlled trials, and observational studies during past influenza seasons and during the 2009 H1N1 pandemic.6-13 Influenza antiviral medications are most effective in treating influenza and reducing complications when treatment is started early (within 48 hours of illness onset). Some studies suggest clinical benefit among hospitalized patients and young children with febrile illness even when treatment was started three to five days after illness onset.14-20
Recommendations for Clinicians
1. All People Six Months and Older Who Have Not Yet Received an Influenza Vaccine this Season Should Be Vaccinated Against Influenza
All available vaccine formulations this season contain A(H3N2), A(H1N1)pdm09, and B/Victoria virus strains.21 The 2019-2020 U.S. quadrivalent influenza vaccines contain these and an additional influenza B/Yamagata virus. CDC does not recommend one influenza vaccine formulation over another.
2. All Hospitalized, Severely Ill, and High-Risk Patients with Suspected or Confirmed Influenza, Regardless of Influenza Vaccination Status, Should Be Treated with Antivirals As Soon As Possible After Onset of Illness
Antiviral treatment is recommended as early as possible for any patient with suspected or confirmed influenza who—
- Is hospitalized.
- Has severe, complicated, or progressive illness. This may include outpatients with severe or prolonged progressive symptoms or patients who develop complications such as pneumonia but who are not hospitalized.
- Is at high risk for influenza complications but not hospitalized. This includes—
- Children younger than two years. Although children younger than five years are considered at higher risk for complications from influenza, the highest risk is for those younger than two years.
- Adults 65 years and
- People with chronic pulmonary (including asthma), cardiovascular (except hypertension alone), renal, hepatic, hematological (including sickle cell disease), and metabolic (including diabetes mellitus)
- People with neurologic and neurodevelopment conditions, including disorders of the brain, spinal cord, peripheral nerve, and muscle, such as cerebral palsy, seizure disorder, stroke, intellectual disability, moderate to severe developmental delay, muscular dystrophy, or spinal cord
- People with immunosuppression, including that caused by medications or by HIV
- Women who are pregnant or postpartum (within two weeks after delivery).
- People younger than 19 years who are receiving long-term aspirin
- American Indians and Alaska
- People with extreme obesity (i.e., body mass index is equal to or greater than 40).
- Residents of nursing homes and other chronic care
3. Antiviral Treatment in Non-High-Risk Patients with Uncomplicated Influenza
Antiviral treatment can benefit other individuals with influenza. While current guidance focuses on antiviral treatment of those with severe illness or at high risk of complications, antiviral treatment may be prescribed for any previously healthy (non-high risk) outpatient with suspected or confirmed influenza who presents within two days after illness onset. Multiple randomized controlled clinical trials (RCTs) and meta-analyses of RCTs have demonstrated efficacy of early initiation of treatment (started within 48 hours of illness onset) with neuraminidase inhibitors in reducing duration of fever and illness symptoms by about a day compared with placebo in otherwise healthy children and adults with uncomplicated influenza.6,9 Clinical judgment—considering the patient’s disease severity and progression, age, likelihood of influenza, and time since onset of symptoms is important when making antiviral treatment decisions for outpatients who are not at increased risk for influenza complications.
4. Choice of Antiviral Medication
Four influenza antiviral medications approved by the U.S. Food and Drug Administration (FDA) are recommended for use in the United States during the 2019-2020 influenza season.
Three drugs are chemically related antiviral medications known as neuraminidase inhibitors: oral oseltamivir phosphate (available as a generic version or under the trade name Tamiflu®), inhaled zanamivir (trade name Relenza®), and intravenous peramivir (trade name Rapivab®). These medications block the viral neuraminidase enzyme and have activity against both influenza A and B viruses.
The fourth drug is oral baloxavir marboxil (trade name Xofluza®), which is active against both influenza A and B viruses but has a different mechanism of action. Baloxavir is a cap-dependent endonuclease inhibitor that interferes with viral RNA transcription and blocks virus replication. In October 2019, FDA approved an indication for baloxavir treatment of acute uncomplicated influenza within two days of illness onset in people 12 years and older at high risk of developing influenza- related complications, based upon the findings of a clinical trial.22 In the clinical trial of early initiation of antiviral treatment for uncomplicated influenza in high-risk adolescents and adults, baloxavir was superior to placebo and had similar overall efficacy to oseltamivir in the time to alleviation of symptoms. There are no available data for baloxavir treatment of influenza in pregnant women, immunocompromised people, those with severe disease, or hospitalized patients.
Recommended ages for treatment and prevention with antiviral medications are summarized in the table below. Dosing and more detailed treatment considerations can be found in the Influenza Antiviral Medications: Summary for Clinicians (https://www.cdc.gov/flu/professionals/antivirals/summary- clinicians.htm).
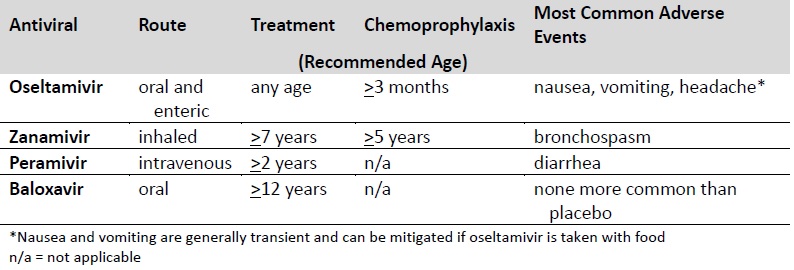
For outpatients with acute uncomplicated influenza, oral oseltamivir, inhaled zanamivir, intravenous peramivir, or oral baloxavir may be used for treatment.
The recommended treatment course for uncomplicated influenza is—
- One dose twice daily of oral oseltamivir for five days, or
- One dose twice daily of inhaled zanamivir for five days, or
- One dose of intravenous peramivir, or
- One dose of oral
Oral or enterically-administered oseltamivir is the only recommended antiviral medication for treating hospitalized patients with suspected or confirmed influenza and patients with severe or complicated illness with suspected or confirmed influenza (e.g., pneumonia, exacerbation of underlying chronic medical condition) who are not hospitalized. Please see the Influenza Antiviral Medications: Summary for Clinicians (https://www.cdc.gov/flu/professionals/antivirals/summary- clinicians.htm) for considerations regarding dosing and duration of antiviral treatment for patients with severe or complicated influenza. There are insufficient data for use of inhaled zanamivir, intravenous peramivir, and oral baloxavir in patients with severe influenza disease.
Oral oseltamivir is preferred for treatment of pregnant women. Oseltamivir has been shown to be safe for treating pregnant women, and pregnant women are recommended to receive the same antiviral dosing as non-pregnant people. Baloxavir is not recommended for treating women or breastfeeding mothers, as there are no available efficacy or safety data.
5. Timing of Treatment and Implications for Patient Evaluation, Treatment, and Testing
Clinical benefit is greatest when antiviral treatment is administered as close to illness onset as possible. Therefore, antiviral treatment should be started as soon as possible and should not wait for laboratory confirmation of influenza. Ideally, treatment should be initiated within 48 hours of onset of symptoms. However, antiviral treatment initiated later than 48 hours after illness onset can still be beneficial for some patients.
A history of current season influenza vaccination does not exclude a diagnosis of influenza in an ill child or adult. High-risk patients should be advised to call their healthcare provider promptly if they have symptoms of influenza.
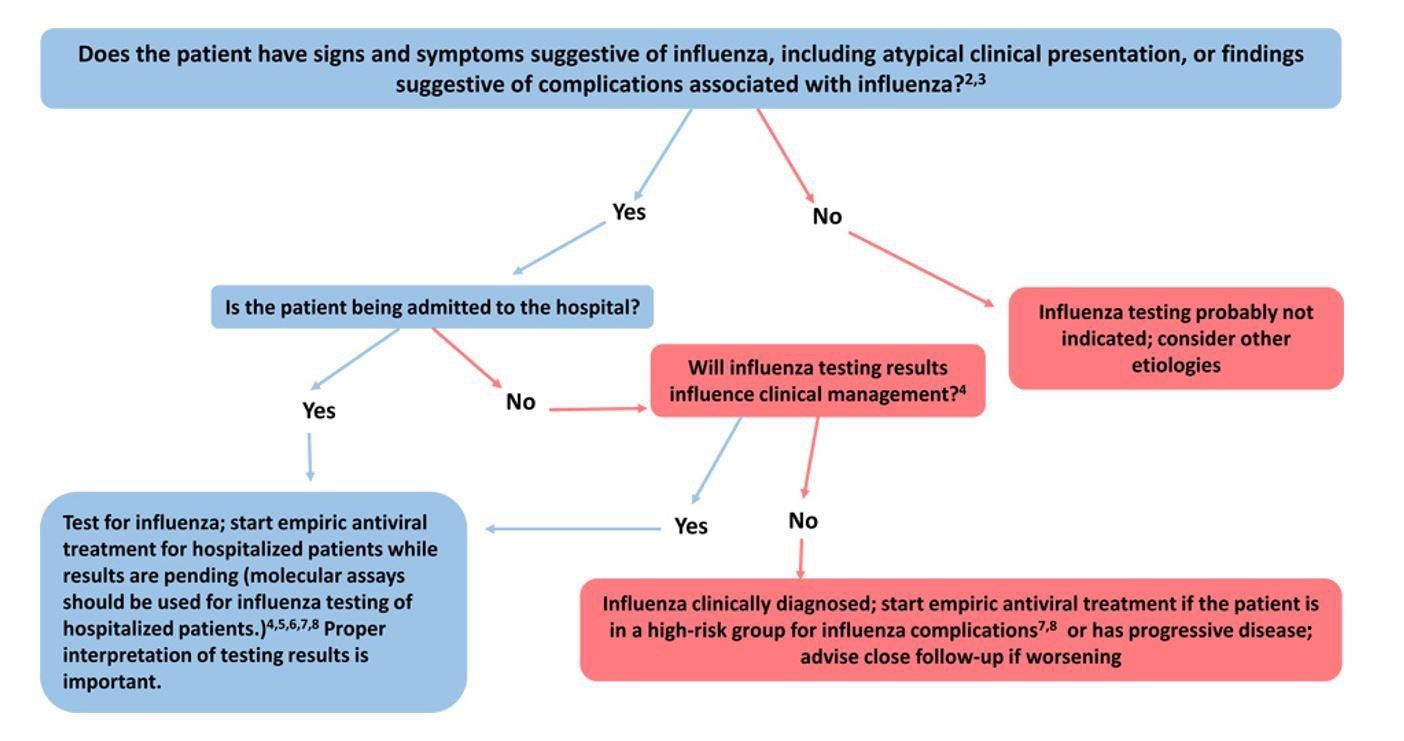
Specimens from all hospitalized patients with suspected influenza should be tested with molecular assays with high sensitivity and specificity since influenza testing can help inform clinical management and prompt implementation of infection prevention and control measures for influenza. Molecular assays (including reverse transcription polymerase chain reaction [RT-PCR]) should be used for influenza testing of hospitalized patients. Treatment should be empiric and should start as soon as possible.23,24 Treatment should not wait for laboratory confirmation.
The following figure may be used as a guide for considering influenza testing (see https://www.cdc.gov/flu/professionals/diagnosis/consider-influenza-testing.htm for footnotes).
Additional influenza resources for health professionals are available on the CDC website at https://www.cdc.gov/flu/professionals. In addition, www.vaccinefinder.org and www.medfinder.org, are available to help individuals find places to get age-appropriate influenza vaccines or fill prescriptions for influenza antivirals.
For More Information
- Weekly U.S. Influenza Surveillance Report, https://www.cdc.gov/flu/weekly/index.htm
- Information for Health Professionals, https://www.cdc.gov/flu/professionals
- Influenza Antiviral Medications: Summary for Clinicians, https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm
- Guide for considering influenza testing when influenza viruses are circulating in the community, https://www.cdc.gov/flu/professionals/diagnosis/consider-influenza-testing.htm
References
Owusu D, Hand J, Tenforde MW, et al. Early Season Pediatric Influenza B/Victoria Virus Infections Associated with a Recently Emerged Virus Subclade — Louisiana, 2019 MMWR Morb Mortal Wkly ePub: 10 January 2020. DOI: http://dx.doi.org/10.15585/mmwr.mm6902e1
- Doyle JD, Campbell AP. Pediatric influenza and illness severity: what is known and what questions remain? Curr Opin Pediatr. 2019;31(1):119-126.
- Shang M, Blanton L, Brammer L, Olsen SJ, Fry AM. Influenza-Associated Pediatric Deaths in the United States, 2010-2016. 2018;141(4).
- Tran D, Vaudry W, Moore D, et al. Hospitalization for Influenza A Versus B. 2016;138(3).
- Budd AP, Beacham L, Smith CB, et al. Birth Cohort Effects in Influenza Surveillance Data: Evidence That First Influenza Infection Affects Later Influenza-Associated Illness. The Journal of infectious diseases. 2019;220(5):820-829.
- Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta- analysis of randomised controlled trials. 2015;385(9979):1729-1737.
- Doll MK, Winters N, Boikos C, Kraicer-Melamed H, Gore G, Quach C. Safety and effectiveness of neuraminidase inhibitors for influenza treatment, prophylaxis, and outbreak control: a systematic review of systematic reviews and/or meta-analyses. J Antimicrob Chemother. 2017;72(11):2990- 3007.
- Hayden FG, Sugaya N, Hirotsu N, et al. Baloxavir Marboxil for Uncomplicated Influenza in Adults and Adolescents. The New England journal of medicine. 2018;379(10):913-923.
- Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and Safety of Oseltamivir in Children: Systematic Review and Individual Patient Data Meta-analysis of Randomized Controlled Trials. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018;66(10):1492-1500.
- Muthuri SG, Venkatesan S, Myles PR, et al. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta- analysis of individual participant data. Lancet Respir Med. 2014;2(5):395-404.
- Santesso N, Hsu J, Mustafa R, et al. Antivirals for influenza: a summary of a systematic review and meta-analysis of observational studies. Influenza Other Respir Viruses. 2013;7 Suppl 2:76- 81.
- Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenzaa. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2019;68(6):895-902.
- Venkatesan S, Myles PR, Leonardi-Bee J, et al. Impact of Outpatient Neuraminidase Inhibitor Treatment in Patients Infected With Influenza A(H1N1)pdm09 at High Risk of Hospitalization: An Individual Participant Data Metaanalysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017;64(10):1328-1334.
- Fry AM, Goswami D, Nahar K, et al. Efficacy of oseltamivir treatment started within 5 days of symptom onset to reduce influenza illness duration and virus shedding in an urban setting in Bangladesh: a randomised placebo-controlled trial. Lancet Infect Dis. 2014;14(2):109-118.
- Lee EH, Wu C, Lee EU, et al. Fatalities associated with the 2009 H1N1 influenza A virus in New York city. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50(11):1498-1504.
- Lee N, Choi KW, Chan PK, et al. Outcomes of adults hospitalised with severe influenza. 2010;65(6):510-515.
- Lee N, Cockram CS, Chan PK, Hui DS, Choi KW, Sung JJ. Antiviral treatment for patients hospitalized with severe influenza infection may affect clinical outcomes. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;46(8):1323- 1324.
- Louie JK, Yang S, Acosta M, et al. Treatment with neuraminidase inhibitors for critically ill patients with influenza A (H1N1)pdm09. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;55(9):1198-1204.
- McGeer A, Green KA, Plevneshi A, et al. Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;45(12):1568-1575.
- Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA : the journal of the American Medical Association. 2010;303(15):1517-1525.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices – United States, 2019-20 Influenza Season. MMWR Recommendations and reports. 2019;68(3):1-21.
- Ison MG PS, Yoshida Y, Shishido T, Hayden F, Uehara T. Phase 3 Trial of Baloxavir Marboxil in High-Risk Influenza Patients (CAPSTONE-2 Study) Late breaker Abstract LB16. Presented on October 6, 2018 at ID Week 2018, San Francisco, CA. . Open Forum Infectious Diseases. 2018;5:S764-S765.
- Katzen J, Kohn R, Houk JL, Ison MG. Early Oseltamivir After Hospital Admission Is Associated With Shortened Hospitalization: A 5-Year Analysis of Oseltamivir Timing and Clinical Outcomes. Clinical infectious diseases : an official publication of the Infectious Diseases Society of 2019;69(1):52-58.
- Venkatesan S, Myles PR, Bolton KJ, et al. Neuraminidase Inhibitors and Hospital Length of Stay: A Meta-analysis of Individual Participant Data to Determine Treatment Effectiveness Among Patients Hospitalized With Nonfatal 2009 Pandemic Influenza A(H1N1) Virus Infection. The Journal of infectious diseases.
The Centers for Disease Control and Prevention (CDC) protects people’s health and safety by preventing and controlling diseases and injuries; enhances health decisions by providing credible information on critical health issues; and promotes healthy living through strong partnerships with local, national, and international organizations.
Categories of Health Alert Network messages:
Health Alert Requires immediate action or attention; highest level of importance
Health Advisory May not require immediate action; provides important information for a specific incident or situation
Health Update Unlikely to require immediate action; provides updated information regarding an incident or situation
HAN Info Service Does not require immediate action; provides general public health information
##This message was distributed to state and local health officers, state and local epidemiologists, state and local laboratory directors, public information officers, HAN coordinators, and clinician organizations##
Go Back